Share this
Keep An Eye On Corrosion And Save Big Money
by Jeff Hopkins on 11/20/19 9:00 AM
Damage to tubing systems is pervasive, problematic and preventable
"Feeling confident that your systems will perform, even in the most demanding locations, involves not only product design but also materials of construction, application-specific corrosion issues, and unique oil field environments."
- From 5 Reasons Northern California Oil & Gas Companies Choose Us and You Should Too
Just about every metal used to build our world corrodes, but only under certain circumstances. If you know how to identify it and where to look for it, you can minimize risk at the refinery and prevent a lot of costly damage.
Let's go through some of the most common forms of corrosion.
Pitting corrosion
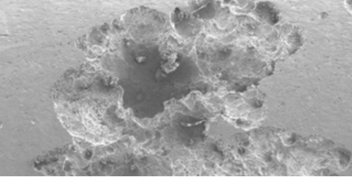 Pitting of a metal occurs when its protective layer breaks down, allowing the exposed metal atoms to give up their electrons easily, resulting in corrosion.
Pitting of a metal occurs when its protective layer breaks down, allowing the exposed metal atoms to give up their electrons easily, resulting in corrosion.
A typical coating on stainless steels and nickel alloys
is a thin chromium-rich oxide film.
This electrochemical reaction causes tiny pits to form. Corrosion products accumulate in the pits, causing the pits to grow deeper. Eventually they may penetrate a tube wall entirely. Pits can also make it easier for cracks to form in stressed components.
When monitoring for corrosion, look for reddish brown iron oxide deposits and for pits, which may have formed, in a metal surface.
Crevice corrosion
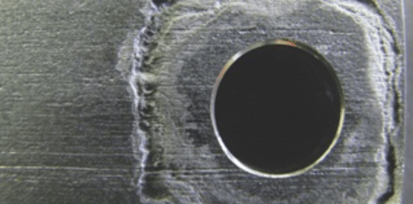 Crevice corrosion likewise starts with the breakdown of the protective oxide film and continues with the formation of shallow pits. But instead of occurring in plain sight, crevice corrosion does what its name implies: It occurs on a metal surface that is in intimate contact with another surface.
Crevice corrosion likewise starts with the breakdown of the protective oxide film and continues with the formation of shallow pits. But instead of occurring in plain sight, crevice corrosion does what its name implies: It occurs on a metal surface that is in intimate contact with another surface.
A common place for crevice corrosion would be where stainless steel tubing is in intimate contact with a tube clamp. You would be able to see it only when a tubing clamp is removed from the installed tubing. It is important to remember that crevice corrosion can occur at lower temperatures than pitting corrosion.
Galvanic corrosion
 Galvanic corrosion occurs when two dissimilar metals come into contact with each other in the presence of an electrolyte, an electrically conducting fluid. Generally speaking, the risk of galvanic corrosion is typically small if the difference in electrochemical potential between the two metals in contact does not exceed 0.2 volts.
Galvanic corrosion occurs when two dissimilar metals come into contact with each other in the presence of an electrolyte, an electrically conducting fluid. Generally speaking, the risk of galvanic corrosion is typically small if the difference in electrochemical potential between the two metals in contact does not exceed 0.2 volts.
Stress corrosion cracking
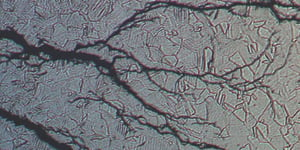 Certain alloys are susceptible to chloride-ion induced stress corrosion cracking. The chloride ion interacts chemically with the material where tensile stresses are highest, such as the very tip of a crack, making it easier for the crack to propagate. This failure mode is dangerous because it can destroy a component at stress levels below the yield strength of an alloy. While in progress, this failure mode can be difficult to detect, and final failure can occur suddenly.
Certain alloys are susceptible to chloride-ion induced stress corrosion cracking. The chloride ion interacts chemically with the material where tensile stresses are highest, such as the very tip of a crack, making it easier for the crack to propagate. This failure mode is dangerous because it can destroy a component at stress levels below the yield strength of an alloy. While in progress, this failure mode can be difficult to detect, and final failure can occur suddenly.
Some alloys are considerably more prone to stress corrosion cracking than others. Three groups of materials are highly resistant to stress corrosion cracking: carbon steels, nickel-based alloys, and duplex stainless steels.
Corrosion Prevention
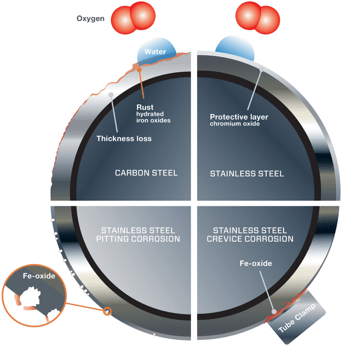 Corrosion often can be minimized by giving your workforce some basic knowledge. First, consider the choice of materials for tubing applications, including tube supports and tube clamps.
Corrosion often can be minimized by giving your workforce some basic knowledge. First, consider the choice of materials for tubing applications, including tube supports and tube clamps.
Our 316 stainless steel tubing works well in many installations so long as it is kept clean and temperatures are not excessively high.
Corrosion of 316 stainless tubing is more likely in hot climates and in installations where rust from carbon steel structural beams and floors accumulates on stainless steel surfaces. For these situations, superaustenitic or superduplex stainless steel offers much better corrosion resistance.
The use of tube support strips should be avoided because the relatively large crevice contact area. The industry is now moving more toward the use of tubing clamps with a design based on minimizing clamp-to-tubing contact, which also facilitates visual inspection of tubing.
Careful system design practices help prevent corrosion by minimizing locations where crevice corrosion can occur and minimizing the contact of non-compatible metals susceptible to galvanic corrosion. Avoid placing tubing directly against walls or against each other.
Beyond these simple measures, maintain a regular and robust corrosion-monitoring program and make sure your workforce gets in-depth training. Swagelok provides classroom learning and hands-on experience that can be invaluable for operators and technicians. Building a basic understanding of corrosion can prevent all manner of problems. Start saving by taking a closer look at your fluid systems—especially where it’s hard to see. Then learn more about our training including our Materials Science Training.
Just ask
Swagelok Northern California has a great deal of exposure to all aspects of fluid system design and engineering. Whether you have a simple question or a complex challenge, we're glad to hear from you.
More like this:
Share this
- Archive (465)
- Assembly Services (207)
- About (100)
- Seal Support Systems (96)
- Best Practices (88)
- Training Services (74)
- Fittings (51)
- Semiconductor Applications (49)
- Hoses and Flexible Tubing (47)
- Regulators (44)
- Tubing (42)
- Grab Sampling Systems (32)
- Sampling Systems (32)
- Gas Systems (30)
- Services (30)
- Downloads (29)
- Valves (24)
- Application Support (18)
- Orbital Welding (17)
- Case Studies (13)
- Steam Systems (13)
- Frequently Asked Questions (12)
- Tools (12)
- Measurement Devices (7)
- Subsystems (6)
- Thermal Management (6)
- September 2023 (1)
- August 2023 (2)
- June 2023 (1)
- March 2023 (3)
- February 2023 (3)
- January 2023 (4)
- December 2022 (4)
- November 2022 (4)
- October 2022 (4)
- September 2022 (1)
- August 2022 (3)
- July 2022 (2)
- June 2022 (4)
- May 2022 (1)
- April 2022 (2)
- March 2022 (1)
- February 2022 (2)
- January 2022 (3)
- December 2021 (1)
- November 2021 (6)
- October 2021 (6)
- September 2021 (8)
- August 2021 (4)
- July 2021 (3)
- June 2021 (6)
- May 2021 (6)
- April 2021 (7)
- March 2021 (5)
- February 2021 (4)
- January 2021 (6)
- December 2020 (5)
- November 2020 (6)
- October 2020 (6)
- September 2020 (8)
- August 2020 (7)
- July 2020 (8)
- June 2020 (8)
- May 2020 (6)
- April 2020 (9)
- March 2020 (7)
- February 2020 (10)
- January 2020 (21)
- December 2019 (23)
- November 2019 (21)
- October 2019 (22)
- September 2019 (21)
- August 2019 (22)
- July 2019 (23)
- June 2019 (20)
- May 2019 (23)
- April 2019 (22)
- March 2019 (21)
- February 2019 (20)
- January 2019 (21)
- December 2018 (14)
- November 2018 (19)
- October 2018 (23)
- September 2018 (17)
- August 2018 (29)
- July 2018 (11)
- June 2018 (6)
- May 2018 (5)
- April 2018 (4)
- March 2018 (5)
- February 2018 (3)
- January 2018 (3)
- December 2017 (2)
- November 2017 (4)
- October 2017 (3)
- September 2017 (2)
- August 2017 (6)
- July 2017 (4)
- June 2017 (4)
- May 2017 (4)
- April 2017 (3)
- March 2017 (4)
- February 2017 (3)
- January 2017 (3)
- December 2016 (3)
- November 2016 (3)
- October 2016 (3)
- September 2016 (5)
- August 2016 (5)
- July 2016 (4)
- June 2016 (5)
- May 2016 (3)
- April 2016 (4)
- March 2016 (5)
- February 2016 (11)
- January 2016 (1)
- December 2015 (3)
- November 2015 (4)
- October 2015 (3)
- September 2015 (4)
- August 2015 (4)
- July 2015 (8)
- June 2015 (5)
- May 2015 (3)
- April 2015 (4)
- March 2015 (4)
- February 2015 (3)
- January 2015 (4)
- December 2014 (2)
- November 2014 (3)
- October 2014 (4)
- September 2014 (4)
- August 2014 (4)
- July 2014 (5)
- June 2014 (4)
- May 2014 (4)
- April 2014 (5)
- March 2014 (4)
- February 2014 (3)
- January 2014 (4)
- December 2013 (5)
- November 2013 (3)
- October 2013 (4)
- September 2013 (3)
- August 2013 (5)
- July 2013 (5)
- June 2013 (5)
- May 2013 (3)
- April 2013 (6)
- March 2013 (4)
- February 2013 (4)
- January 2013 (8)
- December 2012 (4)
- November 2012 (6)
- October 2012 (6)
- September 2012 (4)
- August 2012 (4)
- July 2012 (4)
- June 2012 (4)

.webp?width=210&height=70&name=StickyLogo%20(5).webp)
