Share this
Getting Accurate Results from Analyzers, Part 3: Good Samples
by Jeff Hopkins on 6/20/18 8:30 AM
Deadlegs... permeation... adsorption... all wreak havoc on samples
"Maintaining a representative sample is tricky. There is no alarm that goes off in an analytical system announcing that the sample is unrepresentative. The only way to uncover the problem is to be
familiar with the usual tripping-up points. Fortunately, all of them
are avoidable or correctable."
- Doug Nordstrom, Swagelok Co and Tony Waters, Sampling System Expert
If you want your analytical instrumentation system to give a reliable picture of the fluid in the process line, you need a good sample. If the sample gets altered anywhere in the process, the data from the analyzer won't be useful.
Assuming the sample is properly taken at the tap, it may fall victim to a host of problems:
- Deadlegs or dead spaces that create a “static leak,” where fluid from an old sample might bleed or leak into the new sample
- Contamination, permeation, or adsorption
- A phase change that upsets the balance of chemicals
- A chemical reaction
In this final installment of our three-part series, we'll review the major issues leading to a bad sample and provide recommendations on how to avoid the problems. Also see Getting Accurate Results from Analyzers, Part 1: Time Delays and Getting Accurate Results from Analyzers, Part 2: Calibration.
Deadlegs and dead spaces
It’s important to understand the difference between mixing volumes and deadlegs. They are not quite the same. A mixing volume is a reservoir with a separate inlet and an outlet, such as a filter or knockout pot. Fluid may flow slowly through a mixing volume, but it flows. A deadleg is typically a tee formation with a block at the end so there is no through-flow. Deadlegs can include pressure gauges, transducers, lab sampling valves, and relief valves.
You can calculate the rate at which a mixing volume will flush out an old sample, but that's not true with a deadleg. A deadleg holds the old sample, allowing a small portion of it to mix with the new sample, contaminating it.
Deadlegs are unpredictable. They may eventually clean up or not. Generally, deadlegs become a bigger problem as the ratio of length to diameter increases. In addition, lower flow in the analytical line increases the deadleg’s effect.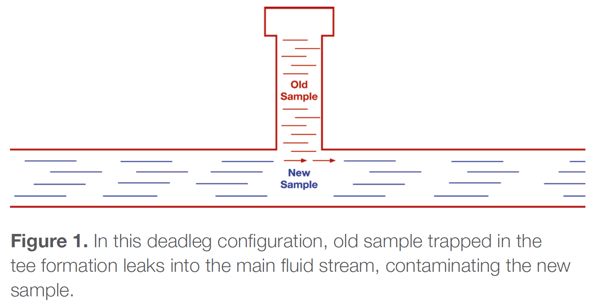
It's best to pick a design that eliminates (or at least minimizes) deadlegs. You can, for instance, remove deadlegs to a bypass loop upstream or downstream of the point where flow is diverted to the analyzer. You also might replace a tee and two-way ball valves with three-way ball valves. You can make sure that a component's end connection minimizes the length of the deadleg. Beyond that, use high flow rates whenever possible.
In addition to deadlegs, some components have “memory.” That is, they have a lot of surface area (such as filters) or permeable materials (some regulators, for example). So instead of putting one filter after the stream selection system, it would be better to purchase multiple filters and locate them before the stream selector system, one in each of the multiple lines.
A less subtle point about component placement concerns the use of the double block and bleed configuration, which consists of two block valves and, in between them, a bleed valve running to a vent. There's a good reason why this has become a standard in the industry: It guards against contamination between fluid streams.
Leaks and permeation
All fluid system components leak because no seal is perfect and all materials are subject to permeation, even stainless steel. In many cases, the leak rate is too slow to matter in an analytical instrumentation system, but not always.
High-quality fluid system components, including valves, are rated to certain temperatures and pressures, and these ratings are published and available. Valves are rated not only for leaks across the seat (internal leaks) but also for shell leaks (external leaks), which are leaks from the inside out.
Permeation is not always an issue. A small amount of oxygen leaking into the sample may not matter, depending on the application. When permeation is a potential issue, the system designer should avoid O-rings, elastomers, and PTFE. Instead, employ stainless steel and metal-to-metal seals wherever possible. Another possibility is to enclose the sampling conditioning system or other parts of the system in a nitrogen-purged box.
Some pneumatic valve designs allow for leaks or permeation between the sample and the actuation air. The valve body and the actuator may be contained in the same block, separated by only a single seal, such as an O-ring. If this single seal were to fail, molecules from the pneumatic air could leak into the sample, or vice versa. Such leaks may lead to a bad analytical reading or, worse, a fire or an explosion. When employing actuators integral to the valve design, look for valves with double seals as well as safety provisions, such as a vented air gap, which allows air or process leaks to safely escape.

Ensuring an Accurate Result in an Analytical Instrumentation System, Part 3: Representative Samples
Adsorption
Adsorption is the tendency of molecules to stick to solid surfaces, including the insides of tubing. Some molecules, like nitrogen and oxygen, are easy to dislodge. Other molecules, like water and hydrogen sulfide, stick to tubing and hold tight. Sticky molecules will not show up in the analytical reading for some time.
If the molecules being measured make up more than 100 ppm of the sample, adsorption will probably not matter a great deal. At a lower concentration, however, adsorption must be addressed. An electropolished surface on the inside of the tubing or a PTFE lining will provide marginal improvements in the adsorption rate. Another option is silicon-lined tubing.

Industrial Sampling Systems, the definitive reference guide by expert Tony Waters (book excerpt)
Phase preservation
Depending on temperature and pressure in the system, molecules in the sample may assume different phases – solids, liquids, or gases, or a mixture of these.
So long as the sample remains all liquid or all gas, the composition will remain the same.
With a two-phase combination of vapor and liquid, however, the vapor and liquid will have different compositions. The analyzer won't be able to determine what the original composition was.
The challenge is to maintain pressure and temperature that will preserve the entire sample in one phase throughout the analytical system. For a gas sample, the simplest solution is to install a regulator, which will lower the pressure. In addition, sample lines can be heated and maintained at the high temperature with insulated, bundled tubing.
Liquid samples are a tougher challenge. A pump can raise the pressure and chillers may be installed. Unfortunately, neither pumps nor chillers are especially easy components to install and maintain.
To read a more detailed discussion of maintaining the integrity of samples, download our free five-page technical paper, Ensuring an Accurate Result in an Analytical Instrumentation System, Part 3: Maintaining a Representative Sample. For an even deeper dive, see the Swagelok Industrial Sampling Systems book (2013), the definitive sampling systems reference guide by expert Tony Waters.

Swagelok Grab Sampling Systems Application Guide (Catalog)
More like this:
- Tony Waters Wrote the Book on Industrial Sampling Systems (blog article)
- Technical Webinar: Pre-Engineered Subsystems (PDF download & webinar replay page)
- More Swagelok technical articles on analytical instrumentation (section of this site)
Share this
- Archive (465)
- Assembly Services (207)
- About (100)
- Seal Support Systems (96)
- Best Practices (88)
- Training Services (74)
- Fittings (51)
- Semiconductor Applications (49)
- Hoses and Flexible Tubing (47)
- Regulators (44)
- Tubing (42)
- Grab Sampling Systems (32)
- Sampling Systems (32)
- Gas Systems (30)
- Services (30)
- Downloads (29)
- Valves (24)
- Application Support (18)
- Orbital Welding (17)
- Case Studies (13)
- Steam Systems (13)
- Frequently Asked Questions (12)
- Tools (12)
- Measurement Devices (7)
- Subsystems (6)
- Thermal Management (6)
- September 2023 (1)
- August 2023 (2)
- June 2023 (1)
- March 2023 (3)
- February 2023 (3)
- January 2023 (4)
- December 2022 (4)
- November 2022 (4)
- October 2022 (4)
- September 2022 (1)
- August 2022 (3)
- July 2022 (2)
- June 2022 (4)
- May 2022 (1)
- April 2022 (2)
- March 2022 (1)
- February 2022 (2)
- January 2022 (3)
- December 2021 (1)
- November 2021 (6)
- October 2021 (6)
- September 2021 (8)
- August 2021 (4)
- July 2021 (3)
- June 2021 (6)
- May 2021 (6)
- April 2021 (7)
- March 2021 (5)
- February 2021 (4)
- January 2021 (6)
- December 2020 (5)
- November 2020 (6)
- October 2020 (6)
- September 2020 (8)
- August 2020 (7)
- July 2020 (8)
- June 2020 (8)
- May 2020 (6)
- April 2020 (9)
- March 2020 (7)
- February 2020 (10)
- January 2020 (21)
- December 2019 (23)
- November 2019 (21)
- October 2019 (22)
- September 2019 (21)
- August 2019 (22)
- July 2019 (23)
- June 2019 (20)
- May 2019 (23)
- April 2019 (22)
- March 2019 (21)
- February 2019 (20)
- January 2019 (21)
- December 2018 (14)
- November 2018 (19)
- October 2018 (23)
- September 2018 (17)
- August 2018 (29)
- July 2018 (11)
- June 2018 (6)
- May 2018 (5)
- April 2018 (4)
- March 2018 (5)
- February 2018 (3)
- January 2018 (3)
- December 2017 (2)
- November 2017 (4)
- October 2017 (3)
- September 2017 (2)
- August 2017 (6)
- July 2017 (4)
- June 2017 (4)
- May 2017 (4)
- April 2017 (3)
- March 2017 (4)
- February 2017 (3)
- January 2017 (3)
- December 2016 (3)
- November 2016 (3)
- October 2016 (3)
- September 2016 (5)
- August 2016 (5)
- July 2016 (4)
- June 2016 (5)
- May 2016 (3)
- April 2016 (4)
- March 2016 (5)
- February 2016 (11)
- January 2016 (1)
- December 2015 (3)
- November 2015 (4)
- October 2015 (3)
- September 2015 (4)
- August 2015 (4)
- July 2015 (8)
- June 2015 (5)
- May 2015 (3)
- April 2015 (4)
- March 2015 (4)
- February 2015 (3)
- January 2015 (4)
- December 2014 (2)
- November 2014 (3)
- October 2014 (4)
- September 2014 (4)
- August 2014 (4)
- July 2014 (5)
- June 2014 (4)
- May 2014 (4)
- April 2014 (5)
- March 2014 (4)
- February 2014 (3)
- January 2014 (4)
- December 2013 (5)
- November 2013 (3)
- October 2013 (4)
- September 2013 (3)
- August 2013 (5)
- July 2013 (5)
- June 2013 (5)
- May 2013 (3)
- April 2013 (6)
- March 2013 (4)
- February 2013 (4)
- January 2013 (8)
- December 2012 (4)
- November 2012 (6)
- October 2012 (6)
- September 2012 (4)
- August 2012 (4)
- July 2012 (4)
- June 2012 (4)

.webp?width=210&height=70&name=StickyLogo%20(5).webp)


