Share this
A Liquid Sample For A Gas Analyzer? Vaporize It
by Jeff Hopkins on 8/8/18 8:30 AM
With a little trial and error and some troubleshooting, you can often make it work
"If the analyzer in your analytical system requires gas but your sample is liquid, the only option is to convert the liquid to gas...Vaporization, done properly, ensures that all of the compounds vaporize at the same time, preserving the sample’s composition."
- Doug Nordstrom, Swagelok Co and Tony Waters, Sampling System Expert
If your sample is liquid and the analyzer in your analytical system requires gas, the only option is to convert the liquid to gas. This process is called vaporization or flash vaporization. As the name implies, you want to turn all the liquid into vapor instantly – without changing the composition.
It's not easy. In fact, sometimes it's not even possible. So make sure it’s really necessary before you try.
Not evaporation
Vaporization is not evaporation. Evaporation occurs gradually with an increase in temperature. Vaporization occurs instantly with a drop in pressure. It’s not possible to vaporize a sample by increasing temperature.
When vaporization is done properly, all of the compounds vaporize at the same time, preserving the sample’s composition. When a mixed sample evaporates, some compounds will evaporate before others, resulting in fractionation. Typically, lighter molecules evaporate first and travel on toward the analyzer, while the heavier molecules remain behind in the liquid phase. That mixed sample is no longer suitable for analysis.
Let’s take a closer look at how to ensure proper vaporization and an accurate analytical result.
Understanding vaporization
The most common tool for the job is a vaporizing regulator, also called a vaporizer. It's a pressure-reducing regulator that can heat the sample at just the right location.
The sample enters the vaporizer as a liquid, then passes through the regulating orifice in the vaporizer. That results in a severe and sudden pressure drop, which vaporizes the liquid. At the same time, heat is applied, which enables the vaporized liquid to remain a vapor. The sample, now a gas, exits the vaporizer and travels to the analyzer.
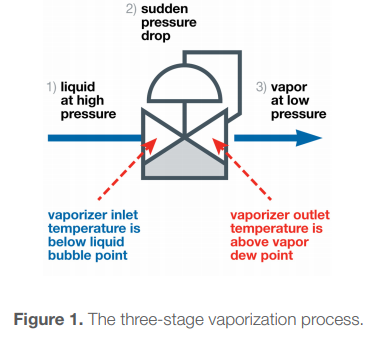
In this delicate process, there are many opportunities for failure. For this short post we'll focus on two main sets of inputs. The first is the composition of the sample. The components may begin to bubble and finish vaporizing at different pressures and temperatures. We’ll need to know what these pressures and temperatures are to successfully manage the process.
The second set of inputs concerns settings of your sampling system: pressure, temperature, and flow. Pressure and temperature are controlled at the vaporizer, while flow is controlled downstream at a rotameter (variable area flowmeter) and needle valve. The first set of inputs tells us what we need to know in order to make the second set of inputs. This takes some trial and error.

How To Manage Vaporization In An Analytical System (5 page Technical Paper)
Understanding your sample
The best way to understand the first set of inputs is with a phase diagram of your system’s particular mixture of compounds. It plots pressure and temperature, and will basically show you three zones. Above the bubble point, the sample will be all liquid. Below the dew point, it’s all vapor. In between is the "no-go zone," the boiling range of the sample. Here, the mixture is part liquid and part vapor, and no longer suitable for analysis. The objective in vaporization is to set the temperature, flow, and pressure so that the sample skips instantly from the liquid side of the no-go zone to the vapor side of the no-go zone.
With pure and nearly pure samples, the no-go zone is tiny. But other samples have such a wide no-go zone that they cannot be successfully vaporized. There's no way to manipulate the variables – temperature, flow, and pressure – in such a way as to avoid fractionation.
Most samples fall between these two extremes.

Industrial Sampling Systems, the definitive reference guide by expert Tony Waters (book excerpt)
Setting temperature, pressure, and flow
In general, at the inlet, you want high pressure and low temperature. At the outlet, you want high temperature and low pressure. But there are limits as to how high and low you can go, and not all of them are completely under your control. Vaporization is basically a balancing act between the variables.
Here is a four-step process for setting your inputs. First, determine the inlet pressure at your vaporizer. This is your process pressure, provided your vaporizer is located close to your sample tap. Higher pressure is better because it allows you to keep the vaporizer temperature higher without boiling the incoming liquid.
Next, set your inlet temperature. You want it low enough that when the sample enters the vaporizer it is entirely a liquid and not bubbling. At the same time, you want it high enough to contribute to the complete flashing of the sample. When you vaporize the sample, the temperature drops, and you don't want it to end up in the boiling range or no-go zone.
The third step is to set the outlet pressure at the vaporizer. Your objective is to drop the pressure below the dew point line. Fourth, set your flow. Flow is set downstream at a valve and rotameter, not at the vaporizer. A high vapor flow moves the sample to the analyzer faster. But with high flow, more heat is required to vaporize the sample. As a rule of thumb, keep the flow rate as low as possible without causing an unacceptable delay in the sample’s travel time to the analyzer.
Another variable is how efficiently the vaporizer transfers heat to the sample. The critical question is how efficiently can the vaporizer replace that heat and keep it flowing to the sample. The more heat the sample can draw, the less its temperature drops during vaporization.
More where that came from
There's a lot more to know about vaporizers than we can pack into a blog post. That's why we have a five-page technical paper that you can download for free. It includes a section on troubleshooting when liquid passes through the vaporizer, and when the sample boils at the inlet.
If you want even more information, see the Swagelok book Industrial Sampling Systems (2013), the definitive sampling systems reference guide by expert Tony Waters.

Swagelok Grab Sampling Systems Application Guide (Catalog)
More like this:
- Tony Waters Wrote the Book on Industrial Sampling Systems (blog article)
- Technical Webinar: Pre-Engineered Subsystems (PDF download & webinar replay page)
- More Swagelok technical articles on analytical instrumentation (section of this site)
Share this
- Archive (465)
- Assembly Services (207)
- About (100)
- Seal Support Systems (96)
- Best Practices (88)
- Training Services (74)
- Fittings (51)
- Semiconductor Applications (49)
- Hoses and Flexible Tubing (47)
- Regulators (44)
- Tubing (42)
- Grab Sampling Systems (32)
- Sampling Systems (32)
- Gas Systems (30)
- Services (30)
- Downloads (29)
- Valves (24)
- Application Support (18)
- Orbital Welding (17)
- Case Studies (13)
- Steam Systems (13)
- Frequently Asked Questions (12)
- Tools (12)
- Measurement Devices (7)
- Subsystems (6)
- Thermal Management (6)
- September 2023 (1)
- August 2023 (2)
- June 2023 (1)
- March 2023 (3)
- February 2023 (3)
- January 2023 (4)
- December 2022 (4)
- November 2022 (4)
- October 2022 (4)
- September 2022 (1)
- August 2022 (3)
- July 2022 (2)
- June 2022 (4)
- May 2022 (1)
- April 2022 (2)
- March 2022 (1)
- February 2022 (2)
- January 2022 (3)
- December 2021 (1)
- November 2021 (6)
- October 2021 (6)
- September 2021 (8)
- August 2021 (4)
- July 2021 (3)
- June 2021 (6)
- May 2021 (6)
- April 2021 (7)
- March 2021 (5)
- February 2021 (4)
- January 2021 (6)
- December 2020 (5)
- November 2020 (6)
- October 2020 (6)
- September 2020 (8)
- August 2020 (7)
- July 2020 (8)
- June 2020 (8)
- May 2020 (6)
- April 2020 (9)
- March 2020 (7)
- February 2020 (10)
- January 2020 (21)
- December 2019 (23)
- November 2019 (21)
- October 2019 (22)
- September 2019 (21)
- August 2019 (22)
- July 2019 (23)
- June 2019 (20)
- May 2019 (23)
- April 2019 (22)
- March 2019 (21)
- February 2019 (20)
- January 2019 (21)
- December 2018 (14)
- November 2018 (19)
- October 2018 (23)
- September 2018 (17)
- August 2018 (29)
- July 2018 (11)
- June 2018 (6)
- May 2018 (5)
- April 2018 (4)
- March 2018 (5)
- February 2018 (3)
- January 2018 (3)
- December 2017 (2)
- November 2017 (4)
- October 2017 (3)
- September 2017 (2)
- August 2017 (6)
- July 2017 (4)
- June 2017 (4)
- May 2017 (4)
- April 2017 (3)
- March 2017 (4)
- February 2017 (3)
- January 2017 (3)
- December 2016 (3)
- November 2016 (3)
- October 2016 (3)
- September 2016 (5)
- August 2016 (5)
- July 2016 (4)
- June 2016 (5)
- May 2016 (3)
- April 2016 (4)
- March 2016 (5)
- February 2016 (11)
- January 2016 (1)
- December 2015 (3)
- November 2015 (4)
- October 2015 (3)
- September 2015 (4)
- August 2015 (4)
- July 2015 (8)
- June 2015 (5)
- May 2015 (3)
- April 2015 (4)
- March 2015 (4)
- February 2015 (3)
- January 2015 (4)
- December 2014 (2)
- November 2014 (3)
- October 2014 (4)
- September 2014 (4)
- August 2014 (4)
- July 2014 (5)
- June 2014 (4)
- May 2014 (4)
- April 2014 (5)
- March 2014 (4)
- February 2014 (3)
- January 2014 (4)
- December 2013 (5)
- November 2013 (3)
- October 2013 (4)
- September 2013 (3)
- August 2013 (5)
- July 2013 (5)
- June 2013 (5)
- May 2013 (3)
- April 2013 (6)
- March 2013 (4)
- February 2013 (4)
- January 2013 (8)
- December 2012 (4)
- November 2012 (6)
- October 2012 (6)
- September 2012 (4)
- August 2012 (4)
- July 2012 (4)
- June 2012 (4)

.webp?width=210&height=70&name=StickyLogo%20(5).webp)


